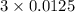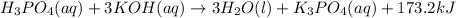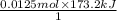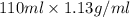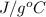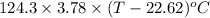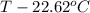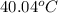Chemistry, 04.03.2020 23:25 Nerdymania
The neutralization of H 3 PO 4 with KOH is exothermic. H 3 PO 4 ( aq ) + 3 KOH ( aq ) ⟶ 3 H 2 O ( l ) + K 3 PO 4 ( aq ) + 173.2 kJ If 55.0 mL of 0.227 M H 3 PO 4 is mixed with 55.0 mL of 0.680 M KOH initially at 22.62 °C, predict the final temperature of the solution, assuming its density is 1.13 g/mL and its specific heat is 3.78 J/(g·°C). Assume that the total volume is the sum of the individual volumes.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 06:30, aurikmah2005
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1

Chemistry, 23.06.2019 12:50, jholbrook7643
Which of these describes the rate of this chemical reaction? h2 + cl2 → 2 hcl a. an increase in the concentration of hcl and h2 with time b. an increase in the concentration of hcl with time c. an increase in h2 and cl2 with time d. a decrease in hcl and cl2 with time
Answers: 1
You know the right answer?
The neutralization of H 3 PO 4 with KOH is exothermic. H 3 PO 4 ( aq ) + 3 KOH ( aq ) ⟶ 3 H 2 O ( l...
Questions in other subjects:




Mathematics, 28.10.2020 09:20

Mathematics, 28.10.2020 09:20

English, 28.10.2020 09:20


Physics, 28.10.2020 09:20

Mathematics, 28.10.2020 09:20

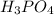 as follows.
as follows.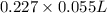
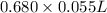
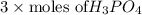 =
=