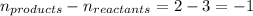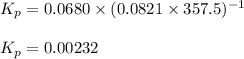Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 20:30, Schoolworkspace453
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
2 SO2(g) + O2(g) 2 SO3(g) Assume that Kc = 0.0680 for the gas phase reaction above. Calculate the co...
Questions in other subjects:


Mathematics, 24.12.2019 03:31


Biology, 24.12.2019 03:31






English, 24.12.2019 03:31

 for this reaction at 84.5°C is 0.00232
for this reaction at 84.5°C is 0.00232 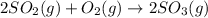
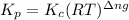
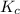 = equilibrium constant in terms of concentration = 0.0680
= equilibrium constant in terms of concentration = 0.0680 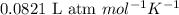
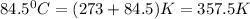
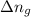 = change in number of moles of gas particles =
= change in number of moles of gas particles =