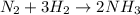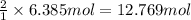Chemistry, 04.03.2020 21:00 hrijaymadathil
In the Haber reaction, patented by German chemist Fritz Haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. This reaction is now the first step taken to make most of the world's fertilizer. Suppose a chemical engineer studying a new catalyst for the Haber reaction finds that liters per second of dinitrogen are consumed when the reaction is run at and. Calculate the rate at which ammonia is being produced.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:30, coralaguilar1702
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 10:30, jetblackcap
An atom that gains or loses one or more electrons is called a(n)
Answers: 1


You know the right answer?
In the Haber reaction, patented by German chemist Fritz Haber in 1908, dinitrogen gas combines with...
Questions in other subjects:

History, 10.07.2019 18:30



Biology, 10.07.2019 18:30

History, 10.07.2019 18:30

Social Studies, 10.07.2019 18:30

Biology, 10.07.2019 18:30



History, 10.07.2019 18:30