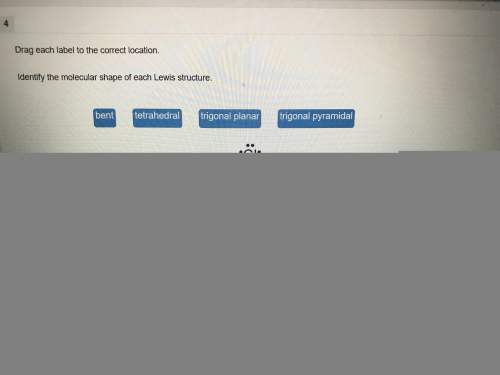
Chemistry, 03.03.2020 20:54 enrique710
A solution of methanol and water has a mole fraction of water of 0.312 and a total vapor pressure of 211 torr at 39.9 ∘C. The vapor pressures of pure methan ol and pure water at this temperature are 256 torr and 55.3 torr, respectively. ls the solution ideal? If not, what can you say about the relative strengths of the solute-solvent interactions compared to the solute-solute and solvent-solvent interactions?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, thechocolatblanc
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 04:30, jocelynmarquillo1
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1


Chemistry, 23.06.2019 09:00, aaronroberson4940
Weight is a measure of: inertia force matter mass
Answers: 1
You know the right answer?
A solution of methanol and water has a mole fraction of water of 0.312 and a total vapor pressure of...
Questions in other subjects:










Business, 27.06.2019 04:50