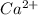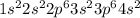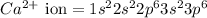Chemistry, 03.03.2020 19:51 Michael845313
The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group 2A According to the octet rule, this element would be expected to form a(n) with a charge of cation anion If X is in period 4, the ion formed has the same electron configuration as the noble gas The symbol for the ion is

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1



Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
The following Lewis diagram represents the valence electron configuration of a main-group element. T...
Questions in other subjects:

Mathematics, 11.11.2020 20:00


Mathematics, 11.11.2020 20:00

English, 11.11.2020 20:00