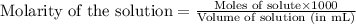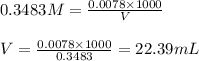Chemistry, 03.03.2020 05:59 egaitapierreval
Oxalic acid is a diprotic acid. If a solid material contains 53.66 percent of oxalic acid (H 2C 2O 4), by mass, then a 0.6543-g sample of that solid will require mL of 0.3483 M NaOH for neutralization. 11.19 97.78 28.59 1.119 22.39

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, daniel1480
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

Chemistry, 23.06.2019 04:00, winterblanco
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1

Chemistry, 23.06.2019 05:30, choatefarmsus
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
Oxalic acid is a diprotic acid. If a solid material contains 53.66 percent of oxalic acid (H 2C 2O 4...
Questions in other subjects:

Mathematics, 29.04.2021 18:50


Mathematics, 29.04.2021 18:50

Mathematics, 29.04.2021 18:50





Mathematics, 29.04.2021 18:50

Chemistry, 29.04.2021 18:50


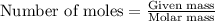


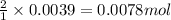 of NaOH
of NaOH