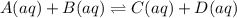A system at equilibrium has two aqueous products and two aqueous reactants. An additional amount of one of the products is added to the system. After the addition, which of the following will change?Select the correct answer below:a. the amount of the added productb. the amount of the other productc. the amounts of the reactantsd. all of the above

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1
You know the right answer?
A system at equilibrium has two aqueous products and two aqueous reactants. An additional amount of...
Questions in other subjects:


Mathematics, 06.07.2019 05:00



Mathematics, 06.07.2019 05:00


Mathematics, 06.07.2019 05:00

History, 06.07.2019 05:00