
A buffer solution that is 0.100 M in both HCOOH and HCOOK has a pH = 3.75. A student says that if a very small amount of 0.100 M HCl is added to the buffer, the pH will decrease by a very small amount. Which of the following best supports the student's claim? (A) HCOO will accept a proton from HCl to produce more HCOOH and H2O(B) HCOOH will accept a proton from HCl to produce more HCOO and H2O. (C) HCOO wil donate a proton to HCl to produce more HCOOH and H2O (D) HCOOH will donate a proton to HCl to produce more HCOO and H2O.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, ayoismeisalex
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 06:40, alyons60
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3
You know the right answer?
A buffer solution that is 0.100 M in both HCOOH and HCOOK has a pH = 3.75. A student says that if a...
Questions in other subjects:


Spanish, 03.02.2020 18:45


English, 03.02.2020 18:45

Mathematics, 03.02.2020 18:45





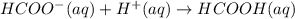
![pk_{a} + log \frac{[HCOO^{-}]}{[HCOOH]}](/tpl/images/0531/9252/8a009.png)
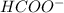 is directly proportional to pH. Hence, when there occurs a decrease in the pH of the solution the
is directly proportional to pH. Hence, when there occurs a decrease in the pH of the solution the ![[HCOO^{-}]](/tpl/images/0531/9252/bee9e.png) will also decrease.
will also decrease. 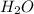 , best supports the student's claim.
, best supports the student's claim.

