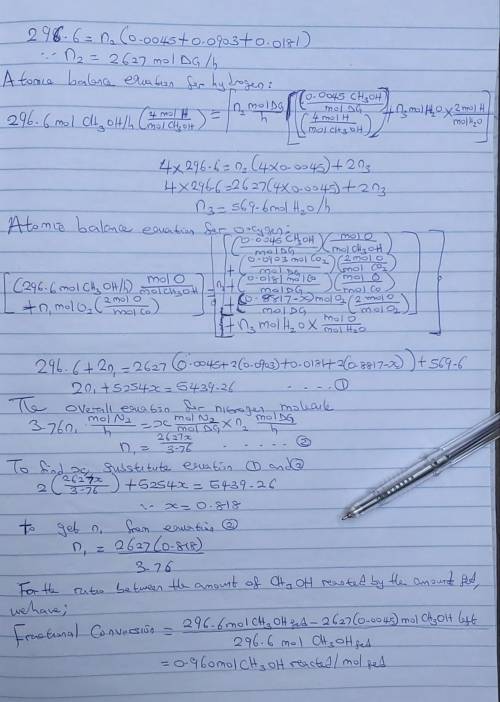
Liquid methanol is fed to a space heater at a rate of 12.0 L/h and burned with excess air. The product gas is analyzed and the following dry-basis mole percentages are determined: CH3OH = 0.45%, CO2 = 9.03%, and CO = 1.81%. (a) After drawing and labeling a flowchart, verify that the system has zero degrees of freedom. (b) Calculate the fractional conversion of methanol, the percentage excess air fed, and the mole fraction of water in the product gas. (c) Suppose the combustion products are released directly into a room. What potential problems do you see and what remedies can you suggest?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, badgirl2005
This is a mixture that has the same composition throughout.
Answers: 1

Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
Liquid methanol is fed to a space heater at a rate of 12.0 L/h and burned with excess air. The produ...
Questions in other subjects:


Mathematics, 13.07.2020 19:01

Mathematics, 13.07.2020 19:01


Computers and Technology, 13.07.2020 19:01

Mathematics, 13.07.2020 19:01



Mathematics, 13.07.2020 19:01