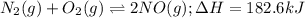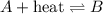Chemistry, 03.03.2020 05:02 emanihackle9
Nitrogen and oxygen react at high temperatures. N2(g) + O2(g) equilibrium reaction arrow 2 NO(g) ΔH = 182.6 kJ (a) What will happen to the concentrations of N2, O2, and NO at equilibrium if more O2 is added?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:40, monkey2865
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3
You know the right answer?
Nitrogen and oxygen react at high temperatures. N2(g) + O2(g) equilibrium reaction arrow 2 NO(g) ΔH...
Questions in other subjects:

Mathematics, 13.04.2021 07:20

Physics, 13.04.2021 07:30





Mathematics, 13.04.2021 07:30


Mathematics, 13.04.2021 07:30

Mathematics, 13.04.2021 07:30