

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 23.06.2019 04:00, winterblanco
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1

Chemistry, 23.06.2019 08:30, elijah4723
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1

Chemistry, 23.06.2019 12:00, Renabelle6350
372 ml is the volume of aluminum, density is 2.70 g/ml what is the mass in grams
Answers: 1
You know the right answer?
Assume that five weak acids, identified only by numbers (1, 2, 3, 4, and 5) have the following ioniz...
Questions in other subjects:

Mathematics, 19.09.2019 05:50

English, 19.09.2019 05:50





History, 19.09.2019 05:50

Biology, 19.09.2019 05:50

Mathematics, 19.09.2019 05:50

![K_a = \frac{[H^+]^2}{[HA]}](/tpl/images/0531/8279/683f8.png)
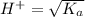 pH = -Log{H⁺]
pH = -Log{H⁺]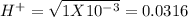 , pH = 1.5
, pH = 1.5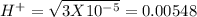 , pH = 2
, pH = 2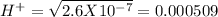 pH = 3
pH = 3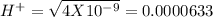 pH = 4
pH = 4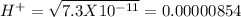 pH = 5
pH = 5 

