Chemistry, 03.03.2020 04:53 GhostElite6383
A 0.9679-g sample containing dimethylphthalate, (194.19 g/mol), and unreactive species was refluxed with 50.00 mL of 0.1215 M to hydrolyze the ester groups (this process is called saponification).
C6H4(COOCH3)2 + 2OH>> C6H4(COO)-2 + H2O
After the reaction was complete, the excess NaOH was back titrated with 32.25mL of 0.1251M HCl. Calculate the percentage of dimethylphthalate in the sample.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:10, aamu15
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 23.06.2019 09:50, jay4881
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1

Chemistry, 23.06.2019 21:30, dunayahsu
Which statement best describes how waves impact beaches? question options: a. beaches change over time as waves move further up the shore. b. beaches change little over time as waves follow the same patterns year after year. c. beaches change little over time as wind patterns stay the same. d. beaches are always changing due to factors such as waves adding and eroding sand.
Answers: 1
You know the right answer?
A 0.9679-g sample containing dimethylphthalate, (194.19 g/mol), and unreactive species was refluxed...
Questions in other subjects:





Business, 29.08.2019 12:00



Health, 29.08.2019 12:00

Computers and Technology, 29.08.2019 12:00

Mathematics, 29.08.2019 12:00

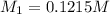
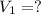

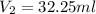
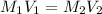
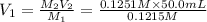
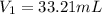
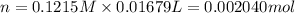

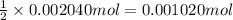 of dimethylphthalate
of dimethylphthalate