
Chemistry, 03.03.2020 03:56 s0cial0bessi0n
Carbon tetrachloride, CCl₄, is a solvent that was once used in large quantities in dry cleaning. Because it is a dense liquid that does not burn, it was also used in fire extinguishers. Unfortunately, its use was discontinued because it was found to be a carcinogen. It was manufactured by the following reaction:
The reaction was economical because the byproduct disulfur dichloride, S₂Cl₂, could be used by industry in the manufacture of rubber products and other materials.
a. What is the percentage yield of CCl₄i if 719 kg is produced from the reaction of 410. kg of CS₂?
b. If 67.5 g of Cl₂ are used in the reaction and 39.5 g of S₂Cl₂ is produced, what is the percentage yield?
c. If the percentage yield of the industrial process is 83.3%, how many kilograms of CS₂ should be reacted to obtain 5.00 × 10⁴ kg of CCl₄? How many kilograms of S₂Cl₂ will be produced, assuming the same yield for that product?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1


Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Carbon tetrachloride, CCl₄, is a solvent that was once used in large quantities in dry cleaning. Bec...
Questions in other subjects:

History, 28.04.2021 04:20

Geography, 28.04.2021 04:20

Mathematics, 28.04.2021 04:20

History, 28.04.2021 04:20




History, 28.04.2021 04:30


History, 28.04.2021 04:30

 = 719/153.82 = 4.674 moles
= 719/153.82 = 4.674 moles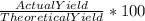 →
→ 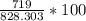 = 86.804 %
= 86.804 %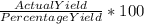 =
=  = 600.24 × 10⁴ kg
= 600.24 × 10⁴ kg

