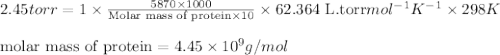Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
The osmotic pressure ofa solution containing 5.87 mg of an unknown protein per 10ml of solution is 2...
Questions in other subjects:




Mathematics, 04.07.2019 01:30



English, 04.07.2019 01:30

Mathematics, 04.07.2019 01:30

Mathematics, 04.07.2019 01:30

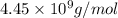
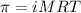
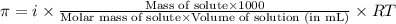
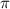 = osmotic pressure of the solution = 2.45 torr
= osmotic pressure of the solution = 2.45 torr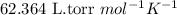
![25^oC=[273+25]=298K](/tpl/images/0531/4710/6a9f9.png)