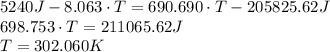A 62.5-g piece of gold at 650. K is dropped into 165 g of H2O (l) at 298 K in an insulated container at 1 bar pressure. Calculate the temperature of the system once equilibrium has been reached. Assume that CP, m for Au and H2O are constant at their values for 298 K throughout the temperature range of interest.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
You know the right answer?
A 62.5-g piece of gold at 650. K is dropped into 165 g of H2O (l) at 298 K in an insulated container...
Questions in other subjects:

Mathematics, 02.02.2021 01:00







Mathematics, 02.02.2021 01:00


Mathematics, 02.02.2021 01:00

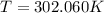
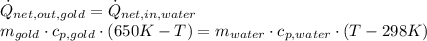
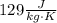 and
and 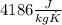 . Last expression is simplified by substituting known variables:
. Last expression is simplified by substituting known variables: