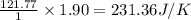Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:30, hcllxxhhlpcj
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 13:30, michealsfamily
The activation energy for a(n) is quite large and usually takes extra energy from the environment, it is normally not a natural spontaneous process. combustion reaction endothermic reaction exothermic reaction catalyzed reaction
Answers: 1
You know the right answer?
Consider the reaction N2(g) + 2O2(g)2NO2(g) Using standard thermodynamic data at 298K, calculate the...
Questions in other subjects:


Chemistry, 21.03.2021 18:30




Mathematics, 21.03.2021 18:30



Mathematics, 21.03.2021 18:30

English, 21.03.2021 18:30

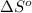 for the surrounding when given amount of nitrogen gas is reacted is 231.36 J/K
for the surrounding when given amount of nitrogen gas is reacted is 231.36 J/K![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_{(product)}]-\sum [n\times \Delta S^o_{(reactant)}]](/tpl/images/0531/3741/52737.png)
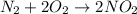
![\Delta S^o_{rxn}=[(2\times \Delta S^o_{(NO_2(g))})]-[(1\times \Delta S^o_{(N_2(g))})+(2\times \Delta S^o_{(O_2(g))})]](/tpl/images/0531/3741/b5946.png)
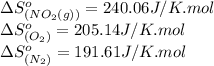
![\Delta S^o_{rxn}=[(2\times (240.06))]-[(1\times (191.61))+(2\times (205.14))]\\\\\Delta S^o_{rxn}=-121.77J/K](/tpl/images/0531/3741/bb1b5.png)