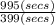An open tubular gas chromatography column is 35.7 m long and has an inner diameter of 0.250 mm. It is coated on the inside wall with a layer of stationary phase that is 1.8 µm (0.0018 mm) thick. Unretained solute, methane, passes through in 6.39 min, whereas toluene has a retention time of 16.35 min. Calculate the retention factor for toluene.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 04:00, hailey200127
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 08:00, mackaylabarnes22
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3

You know the right answer?
An open tubular gas chromatography column is 35.7 m long and has an inner diameter of 0.250 mm. It i...
Questions in other subjects:

History, 21.08.2019 00:30

Mathematics, 21.08.2019 00:30

Mathematics, 21.08.2019 00:30




Biology, 21.08.2019 00:30


World Languages, 21.08.2019 00:30

Chemistry, 21.08.2019 00:30

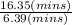 =
=