
Consider the following three processes: (1) Melting of ice at room temperature (2) Boiling of water at 101°C (3) Dissolving of NH4NO3 with water in an instant cold pack? Which statement(s) that are true about ALL three of the given processes? (Choose one or more)(A) Endothermic(B) Exothermic(C) Nonspontaneous(D) Spontaneous(E) none of these statements can be used to describe all three processes

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1


Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
Consider the following three processes: (1) Melting of ice at room temperature (2) Boiling of water...
Questions in other subjects:



History, 20.07.2019 02:30

Mathematics, 20.07.2019 02:30



English, 20.07.2019 02:30


Spanish, 20.07.2019 02:30

Mathematics, 20.07.2019 02:30

 is also an endothermic process. This is because heat is absorbed by water molecules due to which their state changes from liquid to vapor form. When
is also an endothermic process. This is because heat is absorbed by water molecules due to which their state changes from liquid to vapor form. When 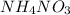 is dissolved in water then heat is absorbed as there occurs a decrease in temperature of water. Hence, it is also an endothermic reaction.
is dissolved in water then heat is absorbed as there occurs a decrease in temperature of water. Hence, it is also an endothermic reaction.

