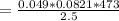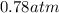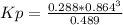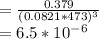Consider the decomposition of the compound C5H6O3 as follows below. C5H6O3(g) → C2H6(g) + 3 CO(g) When a 5.63-g sample of pure C5H6O3(g) was sealed into an otherwise empty 2.50 L flask and heated to 200.°C, the pressure in the flask gradually rose to 1.63 atm and remained at that value. Calculate K for this reaction.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:50, limelight11
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1


Chemistry, 23.06.2019 03:00, amberskids2
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
You know the right answer?
Consider the decomposition of the compound C5H6O3 as follows below. C5H6O3(g) → C2H6(g) + 3 CO(g) Wh...
Questions in other subjects:


History, 09.07.2019 21:30





Geography, 09.07.2019 21:30

Mathematics, 09.07.2019 21:30

Mathematics, 09.07.2019 21:30

Mathematics, 09.07.2019 21:30