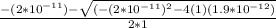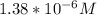Chemistry, 03.03.2020 01:01 ELGuapo6746
Calculate the pH at the equivalence point for the titration of 0.190 M 0.190 M methylamine ( CH 3 NH 2 ) (CH3NH2) with 0.190 M HCl . 0.190 M HCl. The K b Kb of methylamine is 5.0 × 10 − 4 .

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1
You know the right answer?
Calculate the pH at the equivalence point for the titration of 0.190 M 0.190 M methylamine ( CH 3 NH...
Questions in other subjects:

Mathematics, 25.03.2021 22:10

History, 25.03.2021 22:10

French, 25.03.2021 22:10


Health, 25.03.2021 22:10

Social Studies, 25.03.2021 22:10

Chemistry, 25.03.2021 22:10


Social Studies, 25.03.2021 22:10

History, 25.03.2021 22:10

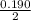
![K_a =\frac{[CH_3NH_2][H^+]}{[CH_3NH^+_3]}](/tpl/images/0531/3181/3871d.png)
![K_a = \frac{[x][x]}{[0.095-x]}](/tpl/images/0531/3181/0ca50.png)
![K_a = \frac{[x^2]}{[0.095-x]}](/tpl/images/0531/3181/85100.png) ------ equation (1)
------ equation (1)
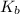 =
=  ; and
; and  ;
;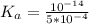
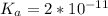




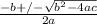 ; we have
; we have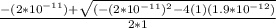 OR
OR