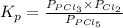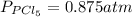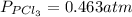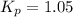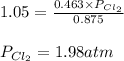Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 02:30, dijonmckenzie3
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
The equilibrium constant for the decomposition of PCl5 at 250 celcius is 1.05. PCl5(g)-->PCl3(g)+...
Questions in other subjects:

History, 23.08.2019 21:20


English, 23.08.2019 21:20



Geography, 23.08.2019 21:20


Health, 23.08.2019 21:20

Biology, 23.08.2019 21:20

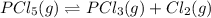
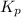 for above reaction follows:
for above reaction follows: