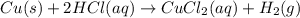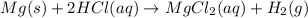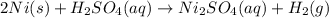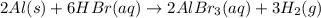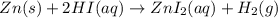Chemistry, 02.03.2020 23:21 raprocksbob
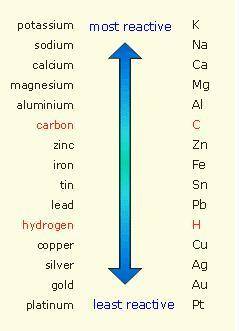
Which of the following reactions is not spontaneous? Cu (s) + 2HCl (aq) → CuCl2 (aq) + H2 (g) Mg (s) + 2HCl (aq) → MgCl2 (aq) + H2(g) 2Ni (s) + H2SO4 (aq) → Ni2SO4 (aq) + H2 (g) 2Al (s) + 6HBr (aq) → 2AlBr3 (aq) + 3H2 (g) Zn (s) + 2HI (aq) → ZnI2(aq) + H2 (g)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2
You know the right answer?
Which of the following reactions is not spontaneous? Cu (s) + 2HCl (aq) → CuCl2 (aq) + H2 (g) Mg (s)...
Questions in other subjects:

Mathematics, 02.05.2021 21:10


Business, 02.05.2021 21:10

Mathematics, 02.05.2021 21:10

Mathematics, 02.05.2021 21:10


Chemistry, 02.05.2021 21:10

Mathematics, 02.05.2021 21:10

Mathematics, 02.05.2021 21:10

Mathematics, 02.05.2021 21:10