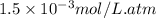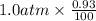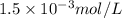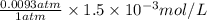Chemistry, 02.03.2020 22:57 brianadee800
Argon makes up 0.93% by volume of air. Calculate its solubility (in mol/L) in water at 20°C and 1.0 atm. The Henry's law constant for Ar under these conditions is 1.5 × 10−3 mol/L·atm.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, markipler01
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2


Chemistry, 23.06.2019 01:20, cedricevans41p4j3kx
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Argon makes up 0.93% by volume of air. Calculate its solubility (in mol/L) in water at 20°C and 1.0...
Questions in other subjects:


English, 03.03.2020 17:26








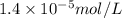
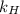 = Henry's law constant of argon =
= Henry's law constant of argon =