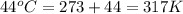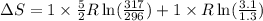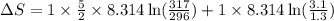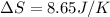Chemistry, 02.03.2020 23:16 camirialchambers17
A sample of helium (He) gas initially at 23°C and 1.0 atm is expanded from 1.3 L to 3.1 L and simultaneously heated to 44°C. Calculate the entropy change for the process.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, kathleendthomas
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
A sample of helium (He) gas initially at 23°C and 1.0 atm is expanded from 1.3 L to 3.1 L and simult...
Questions in other subjects:



English, 06.01.2020 09:31

Chemistry, 06.01.2020 09:31


Social Studies, 06.01.2020 09:31




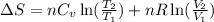
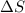 = change in entropy
= change in entropy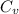 = specific heat capacity at constant volume =
= specific heat capacity at constant volume = 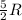 (for monoatomic gas)
(for monoatomic gas)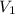 = initial volume of gas = 1.3 L
= initial volume of gas = 1.3 L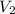 = final volume of gas = 3.1 L
= final volume of gas = 3.1 L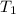 = initial volume of gas =
= initial volume of gas = 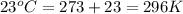
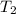 = final volume of gas =
= final volume of gas =