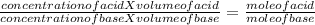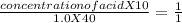Chemistry, 02.03.2020 22:01 sjaybanks4067
During a titration the following data were collected. A 10. mL portion of an unknown monoprotic acid solution was titrated with 1.0 M NaOH; 40. mL of the base were required to neutralize the sample. How many moles of acid are present in 2.0 liters of this unknown solution?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:30, falishaduncanovmtz2
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3


Chemistry, 22.06.2019 07:30, deidaraXneji
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3
You know the right answer?
During a titration the following data were collected. A 10. mL portion of an unknown monoprotic acid...
Questions in other subjects:

English, 28.04.2021 03:30


Mathematics, 28.04.2021 03:30


Mathematics, 28.04.2021 03:30


Mathematics, 28.04.2021 03:30

Mathematics, 28.04.2021 03:30

Mathematics, 28.04.2021 03:30

Mathematics, 28.04.2021 03:30