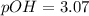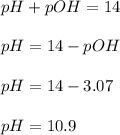Chemistry, 02.03.2020 20:56 eemorales5100
Calculate the pH and pOH of the solutions with the following hydrogen ion or hydroxide ion concentrations. Indicate which solutions are acidic, basic, or neutral. (And please show how to solve! Thanks!) a. [H+]= 3.45x10^-8 M b. [H+]= 2.0x10^-5 M c. [H+]= 7.0x10^-8 M d. [OH-]= 8.56x10^-4 M

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 23.06.2019 00:00, tonimgreen17p6vqjq
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2


Chemistry, 24.06.2019 02:00, RickandMorty420710
What is the best way to compare a concentrated solution to a dilute solution, given the same volume of solution? a. the concentrated solution has less solute than the dilute solution. b. the concentrated solution has the same amount of solute as the dilute solution. c. the concentrated solution has more solute than the dilute solution. d. the concentrated solution has a different amount of solute than the dilute solution. e. the concentrated solution has the same amount of solute as the dilute solution.
Answers: 2
You know the right answer?
Calculate the pH and pOH of the solutions with the following hydrogen ion or hydroxide ion concentra...
Questions in other subjects:

Mathematics, 18.04.2020 07:05



Mathematics, 18.04.2020 07:05

Mathematics, 18.04.2020 07:05

English, 18.04.2020 07:05


Mathematics, 18.04.2020 07:05


![pH=-\log [H^+]](/tpl/images/0530/6113/37e81.png)
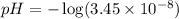
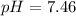

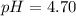

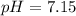
![pOH=-\log [OH^-]](/tpl/images/0530/6113/1fac1.png)