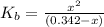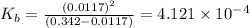Chemistry, 02.03.2020 20:38 mrmendrala
N the laboratory, a general chemistry student measured the pH of a 0.342 M aqueous solution of ethylamine, C2H5NH2 to be 12.067. Use the information she obtained to determine the Kb for this base.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, larreanathalie3523
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2



Chemistry, 23.06.2019 08:30, zhjzjzzj8225
Explain how to convert from one unit to another in the metric system.
Answers: 3
You know the right answer?
N the laboratory, a general chemistry student measured the pH of a 0.342 M aqueous solution of ethyl...
Questions in other subjects:






Mathematics, 21.01.2020 21:31


Social Studies, 21.01.2020 21:31

Biology, 21.01.2020 21:31

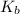 of the an ethylamine is
of the an ethylamine is  .
.![pOH=-\log[OH^-]](/tpl/images/0530/5572/fe336.png)
![1.933=-\log[OH^-]](/tpl/images/0530/5572/b301a.png)
![[OH^-]=0.0117 M](/tpl/images/0530/5572/660e1.png)
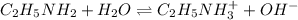
![K_b=\frac{[C_2H_5NH_3^{+}][OH^-]}{[C_2H_5NH_2]}](/tpl/images/0530/5572/63c9d.png)