Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3
You know the right answer?
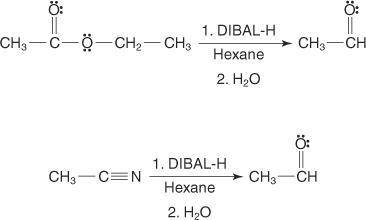
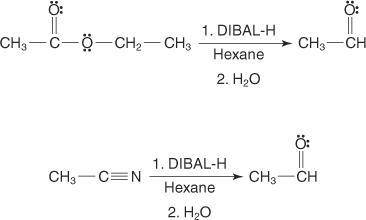
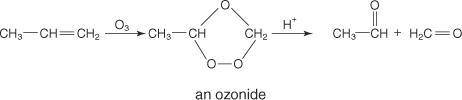
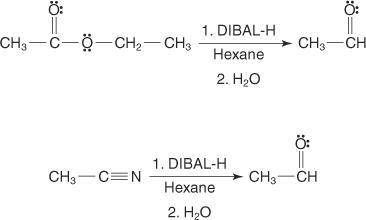
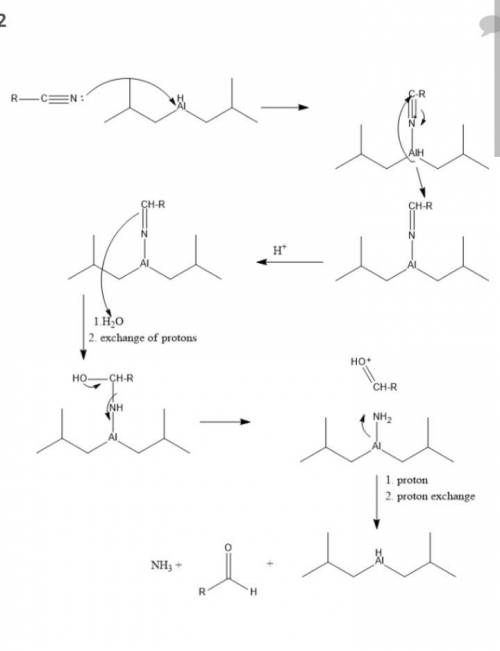
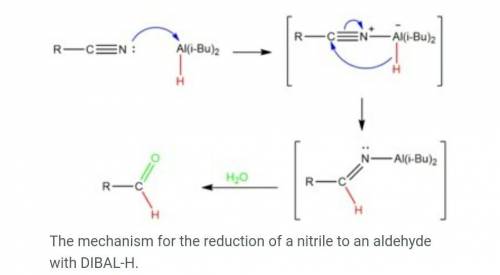
The reagent diisobutylaluminum hydride (DIBALH) reduces esters to aldehydes. When nitriles are treat...
Questions in other subjects:




Advanced Placement (AP), 29.04.2021 06:50


Mathematics, 29.04.2021 06:50


Biology, 29.04.2021 06:50

Mathematics, 29.04.2021 06:50

Advanced Placement (AP), 29.04.2021 06:50