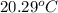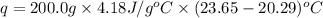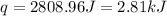A 100.0 mL of 0.500 M HBr at 20.29 oC is added to 100.0 mL of 0.500 M KOH (also at 20.29 oC). After mixing, the temperature rises to 23.65 oC. Calculate the heat of this reaction. [assuming the density and specific heat of HBr and KOH is the same as water, 1.0 g/mL; 4.18 J/g oC, and the volume of the solution is additive].

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, breannaasmith1122
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 07:20, aprilkenedy12
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1

Chemistry, 23.06.2019 09:00, alisonlebron15
Are the results of a thoroughly tested hypothesis?
Answers: 2
You know the right answer?
A 100.0 mL of 0.500 M HBr at 20.29 oC is added to 100.0 mL of 0.500 M KOH (also at 20.29 oC). After...
Questions in other subjects:





English, 27.01.2021 01:40

Biology, 27.01.2021 01:40

Mathematics, 27.01.2021 01:40



Mathematics, 27.01.2021 01:40

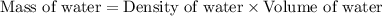
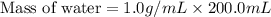
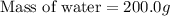
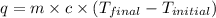
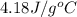
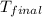 = final temperature =
= final temperature = 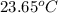
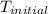 = initial temperature =
= initial temperature =