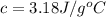Chemistry, 02.03.2020 19:22 PONBallfordM89
A 11.6 g piece of metal is heated to 98°C and dropped into a calorimeter containing 50.0 g of water (specific heat capacity of water is 4.18 J/g°C) initially at 20.5°C. The empty calorimeter has a heat capacity of 125 J/K. The final temperature of the water is 28.2°C. Ignoring significant figures, calculate the specific heat of the metal.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 07:40, wrestling2
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1

Chemistry, 23.06.2019 15:30, tacosloco4629
Light travels through space at 186,282 miles per second and it takes about 1.3 seconds for light to travel from the moon to earth. which of the following is the correct method of finding the distance, in miles, between the moon and earth? add 186,282 and 1.3 divide 186,282 by 1.3 multiply 186,282 by 1.3 subtract 1.3 from 186,282
Answers: 1
You know the right answer?
A 11.6 g piece of metal is heated to 98°C and dropped into a calorimeter containing 50.0 g of water...
Questions in other subjects:



Mathematics, 18.02.2021 22:20

Mathematics, 18.02.2021 22:20



Mathematics, 18.02.2021 22:20



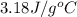
![q=-[q_1+q_2]](/tpl/images/0530/4537/f9283.png)
![m\times c\times (T_f-T_1)=-[c_1\times (T_f-T_2)+m_2\times c_2\times (T_f-T_2)]](/tpl/images/0530/4537/372b8.png)
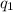 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter = heat absorbed by the water
= heat absorbed by the water = specific heat of calorimeter =
= specific heat of calorimeter = 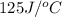
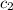 = specific heat of water =
= specific heat of water = 
 = mass of water = 50.0 g
= mass of water = 50.0 g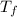 = final temperature =
= final temperature = 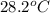
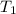 = temperature of metal =
= temperature of metal = 
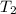 = temperature of water =
= temperature of water = 
![11.6g\times c\times (28.2-98)^oC=-[125J/^oC\times (28.2-20.5)^oC+50.0g\times 4.18J/g^oC\times (28.2-20.5)^oC]](/tpl/images/0530/4537/17b20.png)