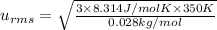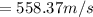Chemistry, 02.03.2020 19:29 firenation18
A 0.50 m3 gas tank holds 3.0 moles of ideal diatomic nitrogen gas at a temperature of 350 K. The atomic mass of nitrogen is 14 g/mol. What is the rms speed of the molecules? (The Boltzmann constant is 1.38 × 10-23 J/K, NA = 6.022 × 1023 molecules/mol.)

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 23.06.2019 04:10, NavyCo
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1
You know the right answer?
A 0.50 m3 gas tank holds 3.0 moles of ideal diatomic nitrogen gas at a temperature of 350 K. The ato...
Questions in other subjects:










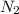 , M= 2 × 14 g/mol = 28 g/mol
, M= 2 × 14 g/mol = 28 g/mol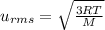
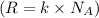
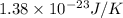
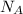 = Avogadro number
= Avogadro number