
Chemistry, 02.03.2020 19:12 chandranewlon
The student determines the molar mass of the gas to be 64 g mol-1. write the expression (set-up) for calculating the percent error in the experimental value, assuming that the unknown gas is butane (molar mass 58 g mol-1). calculations are not required

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 13:30, leeleelynn
If a fast moving car making a loud noise approaches and moves past the person what will happen as the distance between the two increases?
Answers: 1

Chemistry, 23.06.2019 14:00, jamiahfernandes14
What can happen to an atoms electrons when an electric current is passed through the atom?
Answers: 1
You know the right answer?
The student determines the molar mass of the gas to be 64 g mol-1. write the expression (set-up) for...
Questions in other subjects:



Mathematics, 23.10.2021 14:00

English, 23.10.2021 14:00



Arts, 23.10.2021 14:00

Mathematics, 23.10.2021 14:00

SAT, 23.10.2021 14:00

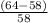 x 100%
x 100%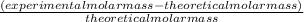 x 100%
x 100%

