c5h12(g)+8o2(g)→5co2(g)+6h2o(g)

Chemistry, 15.10.2019 10:10 ayowazzzgood
The combustion of pentane, c5h12, occurs via the reaction
c5h12(g)+8o2(g)→5co2(g)+6h2o(g)
with heat of formation values given by the following table:
substance δh∘f
(kj/mol)
c5h12 (g) -119.9
co2(g) −393.5
h2o(g) −241.8
calculate the enthalpy for the combustion of 1 mole of pentane.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1


Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
The combustion of pentane, c5h12, occurs via the reaction
c5h12(g)+8o2(g)→5co2(g)+6h2o(g)
c5h12(g)+8o2(g)→5co2(g)+6h2o(g)
Questions in other subjects:



Health, 11.07.2019 10:30

Mathematics, 11.07.2019 10:30


History, 11.07.2019 10:30


History, 11.07.2019 10:30


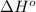
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0321/5182/45485.png)
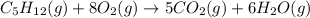
![\Delta H^o_{rxn}=[(n_{(CO_2)}\times \Delta H^o_f_{(CO_2)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})]-[(n_{(C_5H_{12})}\times \Delta H^o_f_{(C_5H_{12})})+(n_{(O_2)}\times \Delta H^o_f_{(O_2)})]](/tpl/images/0321/5182/dfc82.png)
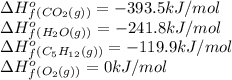
![\Delta H^o_{rxn}=[(5\times -393.5)+(6\times -241.8)]-[(1\times -393.5)+(8\times 0)=-3024.8kJ](/tpl/images/0321/5182/8ec80.png)



