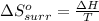Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
I2(g) + Cl2(g)2ICl(g) Using standard thermodynamic data at 298K, calculate the entropy change for th...
Questions in other subjects:



Mathematics, 12.04.2021 04:00

Mathematics, 12.04.2021 04:00


Chemistry, 12.04.2021 04:00

Mathematics, 12.04.2021 04:00

Mathematics, 12.04.2021 04:00


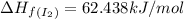

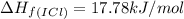
![\Delta H_{rxn}=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0530/3455/db29b.png)
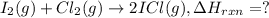
![\Delta H_{rxn}=[(2\times \Delta H_f_{(ICl)})]-[(1\times \Delta H_f_{(I_2)})+(1\times \Delta H_f_{(Cl_2)})]](/tpl/images/0530/3455/4a661.png)
![=[2\times 17.78 kJ/mol]-[1\times 0 kJ/mol+1\times 62.436 kJ/mol]=-26.878 kJ/mol](/tpl/images/0530/3455/6a4a8.png)