Chemistry, 02.03.2020 18:22 hsjsjsjdjjd
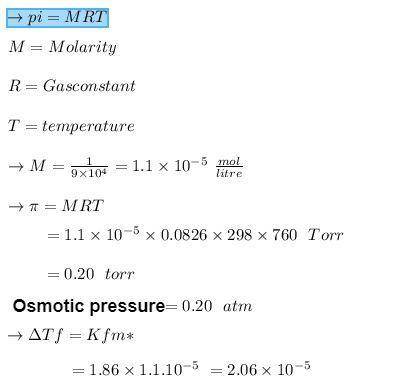
Calculate the freezing-point depression and osmotic pressure at 258C of an aqueous solution containing 1.0 g/L of a protein (molar mass 5 9.0 3 104 g/mol) if the density of the solution is 1.0 g/cm3. b. Considering your answer to part a, which colligative property, freezing-point depression or osmotic pres- sure, would be better used to determine the molar masses of large molecules

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, Mercedes12152002
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1


Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Calculate the freezing-point depression and osmotic pressure at 258C of an aqueous solution containi...
Questions in other subjects:



Spanish, 01.11.2019 11:31




English, 01.11.2019 11:31


Mathematics, 01.11.2019 11:31