

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1


Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Calculate the pH and fraction of dissociation ( α ) for each of the acetic acid ( CH 3 COOH , p K a...
Questions in other subjects:

Mathematics, 05.07.2019 09:00

Biology, 05.07.2019 09:00

Mathematics, 05.07.2019 09:00


History, 05.07.2019 09:00

History, 05.07.2019 09:00

Mathematics, 05.07.2019 09:00



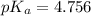
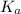
![pK_a=-\log[K_a]](/tpl/images/0530/3712/78bbf.png)
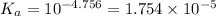
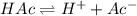
![K_a=\frac{[H^+][Ac^-]}{[HAc]}](/tpl/images/0530/3712/ce0d8.png)
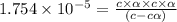
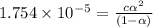
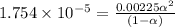
![[H^+]=c\alpha = 0.00225M\times 0.08448=0.0001901 M](/tpl/images/0530/3712/81253.png)
![pH=-\log[H^+]](/tpl/images/0530/3712/cf945.png)
![=-\log[0.0001901 M]=3.72](/tpl/images/0530/3712/5ff4a.png)


