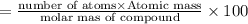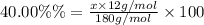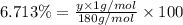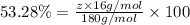Chemistry, 02.03.2020 18:30 leylaanderson85311
A compound consists of 40.00% C, 6.713% H and 53.28% O on a mass basis and has a molar mass of about 180 g/mole. Determine the molecular formula of the compound.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 17:30, kiaramccurty
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
A compound consists of 40.00% C, 6.713% H and 53.28% O on a mass basis and has a molar mass of about...
Questions in other subjects:


Mathematics, 22.04.2021 14:00

Chemistry, 22.04.2021 14:00

Arts, 22.04.2021 14:00


Mathematics, 22.04.2021 14:00


Computers and Technology, 22.04.2021 14:00

Computers and Technology, 22.04.2021 14:00

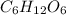 .
.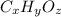 .in
.in