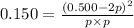Chemistry, 02.03.2020 18:30 justkevin1231
In the chemical reaction:Br2(g) + Cl2(g) ⇌ 2 BrCl(g) KP = 0.150If there is initially 0.500 atm of BrCl and nothing else in a container, what would be the equilibrium concentration of BrCl?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2


Chemistry, 23.06.2019 01:00, stefaniethibodeaux
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
In the chemical reaction:Br2(g) + Cl2(g) ⇌ 2 BrCl(g) KP = 0.150If there is initially 0.500 atm of Br...
Questions in other subjects:

Mathematics, 17.11.2020 01:30

Physics, 17.11.2020 01:30

Mathematics, 17.11.2020 01:30

Mathematics, 17.11.2020 01:30

Health, 17.11.2020 01:30


Mathematics, 17.11.2020 01:30

History, 17.11.2020 01:30


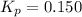
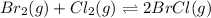
![K_p=\frac{[BrCl]^2}{[Br_2][Cl_2]}](/tpl/images/0530/3756/5c4f9.png)