
Chemistry, 02.03.2020 18:29 mariahisawsome1
Manganese commonly occurs in nature as a mineral. The extraction of manganese from the carbonite mineral rhodochrosite, involves a two-step process. In the first step, manganese (II) carbonate and oxygen react to form manganese (IV) oxide and carbon dioxide: 2 MnCO3(s) + O2(g) → 2MnO2(s) + 2CO2(g) In the second step, manganese (IV) oxide and aluminum react to form manganese and aluminum oxide: 3 MnO2(s) + 4 Al(s) → 3 Mn(s) + 2 Al2O3(s) Write the net chemical equation for the production of manganese (II) carbonate, oxygen and aluminum. Be sure your equation is balanced.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
Manganese commonly occurs in nature as a mineral. The extraction of manganese from the carbonite min...
Questions in other subjects:

Social Studies, 30.03.2021 04:20

Mathematics, 30.03.2021 04:20

Computers and Technology, 30.03.2021 04:20

Mathematics, 30.03.2021 04:20

Mathematics, 30.03.2021 04:20

Spanish, 30.03.2021 04:30



Mathematics, 30.03.2021 04:30

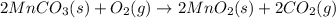
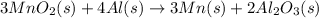
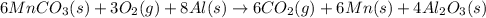
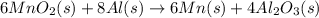
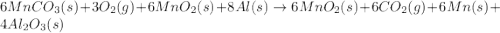
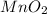 is common on both side, by cancelling it, we get:
is common on both side, by cancelling it, we get:

