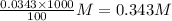Chemistry, 02.03.2020 17:56 trodrickwilliams2019
Determine the molar concentration of ammonium ions, NH4 , in a solution that results when 4.53 g of (NH4)2SO4 are dissolved in 100 mL of water.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, board1692
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2
You know the right answer?
Determine the molar concentration of ammonium ions, NH4 , in a solution that results when 4.53 g of...
Questions in other subjects:



Mathematics, 11.04.2020 23:10


History, 11.04.2020 23:10


Mathematics, 11.04.2020 23:10

History, 11.04.2020 23:10

Biology, 11.04.2020 23:10

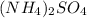 is 132.14 g/mol
is 132.14 g/mol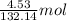 of
of