
Chemistry, 02.03.2020 17:29 ericchen4399
The value of Δ G ° ' for the conversion of glucose-6-phosphate to fructose-6-phosphate (F6P) is + 1.67 kJ/mol . If the concentration of glucose-6-phosphate at equilibrium is 2.95 mM , what is the concentration of fructose-6-phosphate? Assume a temperature of 25.0 ° C .

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, carsonjohnsonn
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 07:00, misspicafunpoke
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
The value of Δ G ° ' for the conversion of glucose-6-phosphate to fructose-6-phosphate (F6P) is + 1....
Questions in other subjects:




Physics, 01.04.2020 19:45


Mathematics, 01.04.2020 19:45


Biology, 01.04.2020 19:45


Mathematics, 01.04.2020 19:45

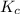
![K_c=\frac{[\text{Fructose-6-phosphate}]}{[\text{Glucose-6-phosphate}]}](/tpl/images/0530/2012/7727c.png)
![K_c=\frac{[\text{Fructose-6-phosphate}]}{2.95 mM}](/tpl/images/0530/2012/f71d2.png)
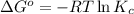
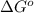 = standard Gibbs free energy = 1.67 kJ/mol = 1670 J/mol (Conversion factor: 1kJ = 1000J)
= standard Gibbs free energy = 1.67 kJ/mol = 1670 J/mol (Conversion factor: 1kJ = 1000J)
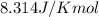
![25.0^oC=[273+25.0]K=298.0 K](/tpl/images/0530/2012/fee79.png)
![1670 J/mol=-8.314 J/mol K\times 298.0 K\times \ln \frac{[\text{Fructose-6-phosphate}]}{2.95 mM}](/tpl/images/0530/2012/2fb29.png)
![[\text{Fructose-6-phosphate}]=1.503 mM](/tpl/images/0530/2012/8d092.png)


