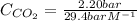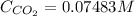Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, anamaliiow
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
The Henry's law constant for CO 2 ( g ) CO2(g) in water at 25 ∘ C 25 ∘C is 29.4 bar⋅M − 1 29.4 bar·M...
Questions in other subjects:

Social Studies, 05.12.2020 03:50


Mathematics, 05.12.2020 03:50

Mathematics, 05.12.2020 03:50





Biology, 05.12.2020 03:50

Social Studies, 05.12.2020 03:50

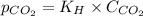
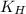 = Henry's constant =
= Henry's constant = 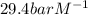
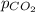 = partial pressure of carbonated drink = 2.20 bar
= partial pressure of carbonated drink = 2.20 bar