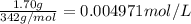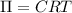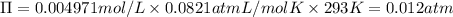Chemistry, 02.03.2020 16:58 gjeaneasley
Wastewater discharged into a stream by a sugar refinery contains 1.70 g of sucrose (C12H22O11) per liter. A government-industry project is designed to test the feasibility of removing the sugar by reverse osmosis. What pressure must be applied to the apparatus at 20.°C to produce pure water?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2


Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Wastewater discharged into a stream by a sugar refinery contains 1.70 g of sucrose (C12H22O11) per l...
Questions in other subjects:

Physics, 29.08.2019 13:30

French, 29.08.2019 13:30




Arts, 29.08.2019 13:30

History, 29.08.2019 13:30

Biology, 29.08.2019 13:30

Health, 29.08.2019 13:30

Mathematics, 29.08.2019 13:30