
Chemistry, 02.03.2020 17:06 aariannahnorwoo
At a particular temperature, K = 4.1 ✕ 10−6 for the following reaction. 2 CO2(g) 2 CO(g) + O2(g) If 2.3 moles of CO2 is initially placed into a 4.9-L vessel, calculate the equilibrium concentrations of all species.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
At a particular temperature, K = 4.1 ✕ 10−6 for the following reaction. 2 CO2(g) 2 CO(g) + O2(g) If...
Questions in other subjects:

Chemistry, 22.07.2019 15:30

Mathematics, 22.07.2019 15:30

Mathematics, 22.07.2019 15:30


History, 22.07.2019 15:30

Social Studies, 22.07.2019 15:30


History, 22.07.2019 15:30

Mathematics, 22.07.2019 15:30

![[O_2]](/tpl/images/0530/1146/b0db0.png) = 0.0124 = 12.4 ×10⁻³ M
= 0.0124 = 12.4 ×10⁻³ M![[CO]](/tpl/images/0530/1146/32558.png) = 0.0248 = 2.48 ×10⁻² M
= 0.0248 = 2.48 ×10⁻² M![[CO_2]](/tpl/images/0530/1146/5494d.png) = 0.4442 M
= 0.4442 M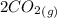 ⇄
⇄ 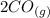 +
+ 
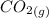 =
= 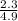 = 0.469
= 0.469![\frac{[CO]^2[O]}{[CO_2]^2}](/tpl/images/0530/1146/9940b.png)
![\frac{[2x]^2[x]}{[0.469-2x]^2}](/tpl/images/0530/1146/25de6.png)
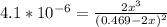
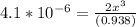

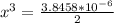
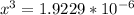
![x=\sqrt[3]{1.9929*10^{-6}}](/tpl/images/0530/1146/6ad64.png)



