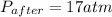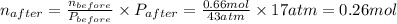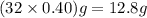A rigid tank contains 0.66 mol of oxygen (O2). Find the mass of oxygen that must be withdrawn from the tank to lower the pressure of the gas from 43 atm to 17 atm. Assume that the volume of the tank and the temperature of the oxygen are constant during this operation. Answer in units of g.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
A rigid tank contains 0.66 mol of oxygen (O2). Find the mass of oxygen that must be withdrawn from t...
Questions in other subjects:

Mathematics, 27.04.2021 14:00

Mathematics, 27.04.2021 14:00


Computers and Technology, 27.04.2021 14:00




Social Studies, 27.04.2021 14:00

History, 27.04.2021 14:00

Mathematics, 27.04.2021 14:00

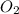 must be withdrawn from tank
must be withdrawn from tank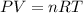
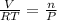
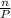 ratio will also be constant before and after removal of
ratio will also be constant before and after removal of 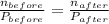
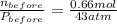 and
and