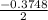Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, janelisse199820
Non renewable resources like petroleum eventually
Answers: 2


Chemistry, 22.06.2019 21:00, melissalopez12
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
Calculate the number of moles of Cl2 produced at equilibrium when 3.98 mol of PCl5 is heated at 283....
Questions in other subjects:










Mathematics, 20.09.2020 06:01

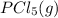 ⇄
⇄ 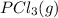 +
+ 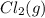
![\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0529/9897/247b7.png)
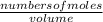
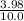
![\frac{[x][x]}{[0.398-x]}](/tpl/images/0529/9897/2afb6.png)
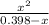
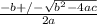
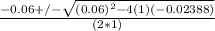
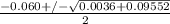
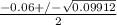
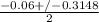
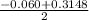 or
or 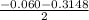
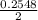 or
or