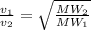Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Balloon X is filled with the carbon monoxide. Balloon Y is filled to the same volume with carbon dio...
Questions in other subjects:

English, 20.01.2022 19:50




Computers and Technology, 20.01.2022 19:50

Physics, 20.01.2022 19:50



Computers and Technology, 20.01.2022 19:50