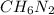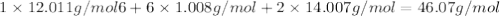Chemistry, 02.03.2020 07:15 snowprincess99447
Determine the molecular formula for the compound with a molar mass of 46.08 g/mol and the following percent compostion:
C:H:N:26.06%13.13%60.81%

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

You know the right answer?
Determine the molecular formula for the compound with a molar mass of 46.08 g/mol and the following...
Questions in other subjects:

English, 09.04.2021 03:20

Mathematics, 09.04.2021 03:20

Biology, 09.04.2021 03:20

Mathematics, 09.04.2021 03:20


Mathematics, 09.04.2021 03:20



Mathematics, 09.04.2021 03:20