

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2


Chemistry, 22.06.2019 16:30, Kathryn014
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
A molecule has the empirical formula C4H6O. If its molecular weight is determined to be about 212 g/...
Questions in other subjects:

Mathematics, 14.11.2020 20:10



Mathematics, 14.11.2020 20:10

English, 14.11.2020 20:10

Mathematics, 14.11.2020 20:10


Mathematics, 14.11.2020 20:10

History, 14.11.2020 20:10

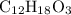 .
. 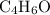 , then the molecular formula would be
, then the molecular formula would be 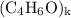 , or equivalently
, or equivalently 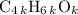 , where
, where 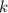 is a positive whole number (
is a positive whole number (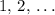 , etc.) The goal here is to find the value of
, etc.) The goal here is to find the value of 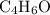 would be
would be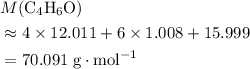 .
.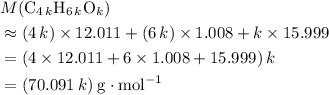 .
.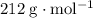 ,
, 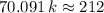 .
. . (Round to the nearest whole number.)
. (Round to the nearest whole number.)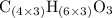 , which simplifies to
, which simplifies to 

