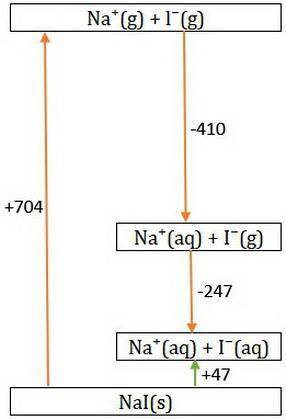
Consider Nal → Na+ + - and the following information.
Hlat = -704 kJ/mol
AHhydr of Na+=...

Chemistry, 01.03.2020 00:17 2022maldonadoleonel
Consider Nal → Na+ + - and the following information.
Hlat = -704 kJ/mol
AHhydr of Na+= -410.0 kJ/mol
AHhydr of -= -247 kJ/mol
What is the AHSol of this compound? Use AHsol = -AHlat + AHhydr.
0-867 kJ/mol
|-867.0 kJ/mol
0 47 kJ/mol
0 47.0 kJ/mol

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, ladypink94
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 21.06.2019 18:10, hannacarroll2539
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Questions in other subjects:



Mathematics, 22.09.2019 06:30

Chemistry, 22.09.2019 06:30

Chemistry, 22.09.2019 06:30


Chemistry, 22.09.2019 06:30


Mathematics, 22.09.2019 06:30

Social Studies, 22.09.2019 06:30